Non-healing chronic wounds are a significant problem, particularly in aged care. A protein called TNF-α, was found to be elevated in these wounds. An antibody specific to TNF-α, is available (Infliximab) and is effective in reducing TNF-α and promoting wound healing. However, ingesting high-doses of Infliximab, can produce dangerous side effects that require treatment to be discontinued. An effective system for delivering Infliximab directly to wounds should overcome this problem.
Dr Steven McInnes, Prof. Nicolas Voelcker and colleagues at the University of South Australia (UniSA) have developed porous silicon (pSi) that can be easily engineered with various structures and pore sizes. They used the ToF-SIMS in the AMMRF (now Microscopy Australia) at UniSA to evaluate and optimise the structures for their ability to carry large quantities of therapeutic molecules, including Infliximab.
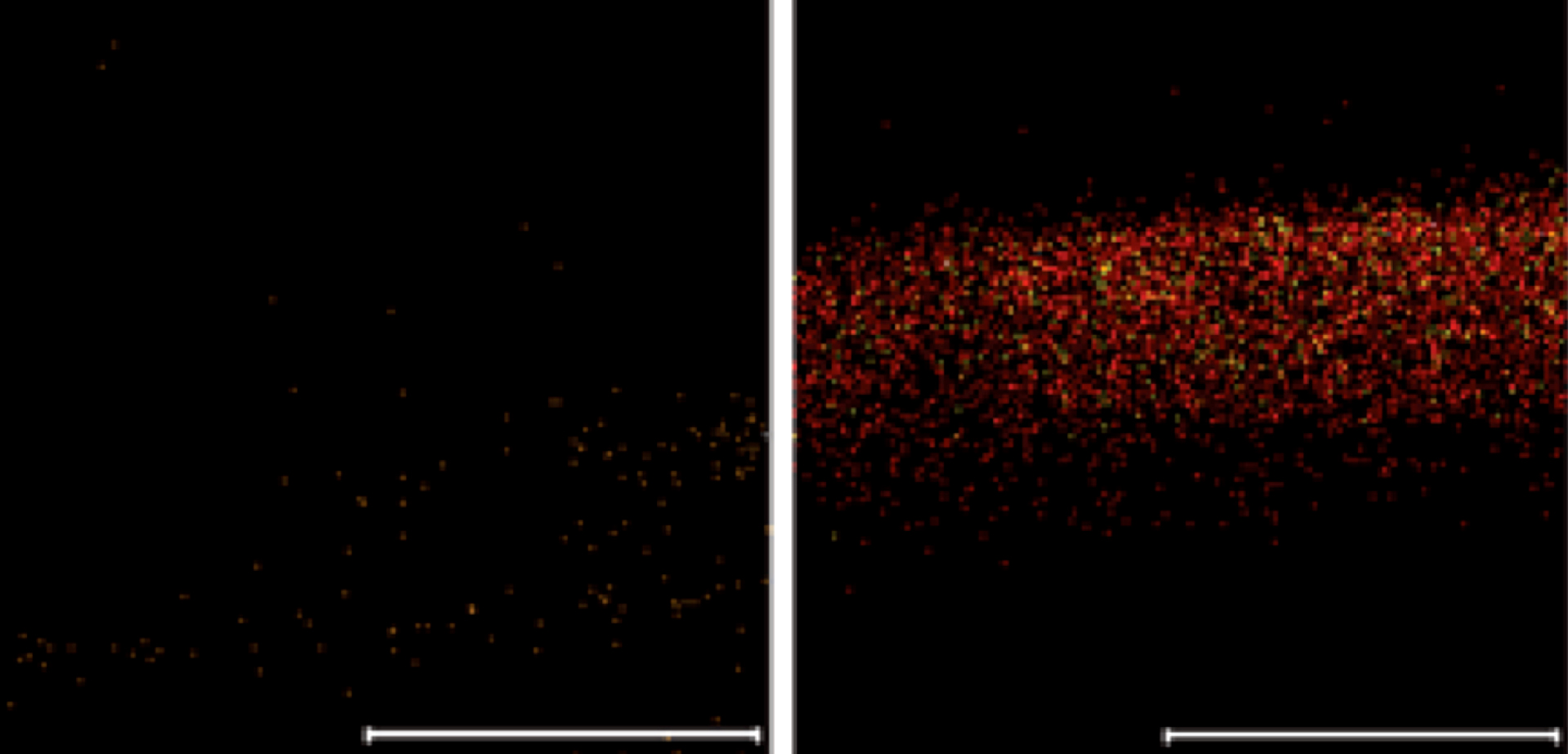
ToF-SIMS image showing porous silicon before and after loading with Infliximab protein. The red and green spots indicate the presence of two distinct fragments diagnostic of protein. Scale bars are 100 μm.
They can also control the charge on the particles, which helps regulate the rate of protein release. When they tested their engineered particles for the controlled release of Infliximab in simulated chronic wound environments they found a steady release from the pSi microparticles over at least 8 days. The released antibody remained able to neutralize TNF-α.
These results suggest that the pSi delivery vehicle is suitable for clinical applications in chronic wound therapy. Future work will focus on the development of pSi nanoparticle delivery systems for incorporation into topical creams and bandages.
Array
October 23, 2015