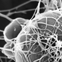
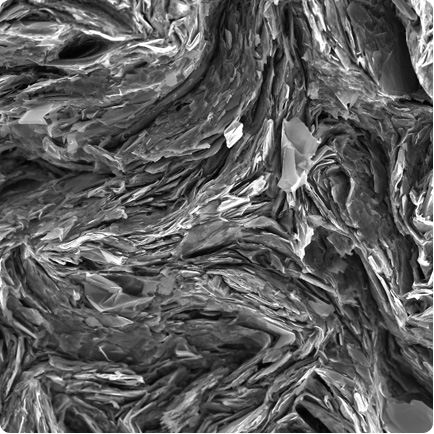
 When is lead not lead? – when it's carbon! This image of graphite from an ordinary pencil 'lead', shows the flakey layers that mark the paper when you write. Graphite's slippery carbon structure also makes it really effective as a lubricant. It is also the basis for a lot of nanotechnology – a very useful substance.
When is lead not lead? – when it's carbon! This image of graphite from an ordinary pencil 'lead', shows the flakey layers that mark the paper when you write. Graphite's slippery carbon structure also makes it really effective as a lubricant. It is also the basis for a lot of nanotechnology – a very useful substance.
Visualised using scanning electron microscopy by John Terlet, University of Adelaide.
Size: 80 x 80 micrometre area.
Graphite is a form of carbon where the atoms join together in flat rings that slip over each other easily. It is one form of pure carbon. Another is diamond where the atoms are all attached to each other in a much stronger way – you can't wipe bits of diamond off a diamond ring with a piece of paper. Graphite's flakey structure also helps it to be a good lubricant at very high temperatures. Different grades of graphite have different sized flakes.