There is no synthetic bone scaffold strong enough to grow large, load-bearing bones such as the spine and leg bones.
When large areas of bone are damaged, surgeons insert porous templates to guide the growth of regenerating bone tissue. Current bone scaffolds have a variety of issues:
Large bone defects and loss due to cancer or trauma can result in scar tissue that impairs the bones’ ability to repair and regenerate even with a scaffold. To encourage growth a transplant of the patients own bone is used. This has several complications including risk of chronic pain and morbidity at the donor site.
A 3D printable ceramic bone scaffold, with the strength and elasticity of healthy bone, has been developed by Prof. Hala Zreiqat’s team at the University of Sydney. They incorporated trace elements that are important in bone formation into a biocompatible ceramic. This material was then tweaked mechanically and chemically to make it 100 times stronger than the bone substitutes in clinical use today. The scaffold slowly dissolves into the body as the bone regenerates.
Recently the researchers have also developed a new method that can turn patient skin cells into bone cells. These have the potential to be embedded into the bone scaffold to facilitate healing.
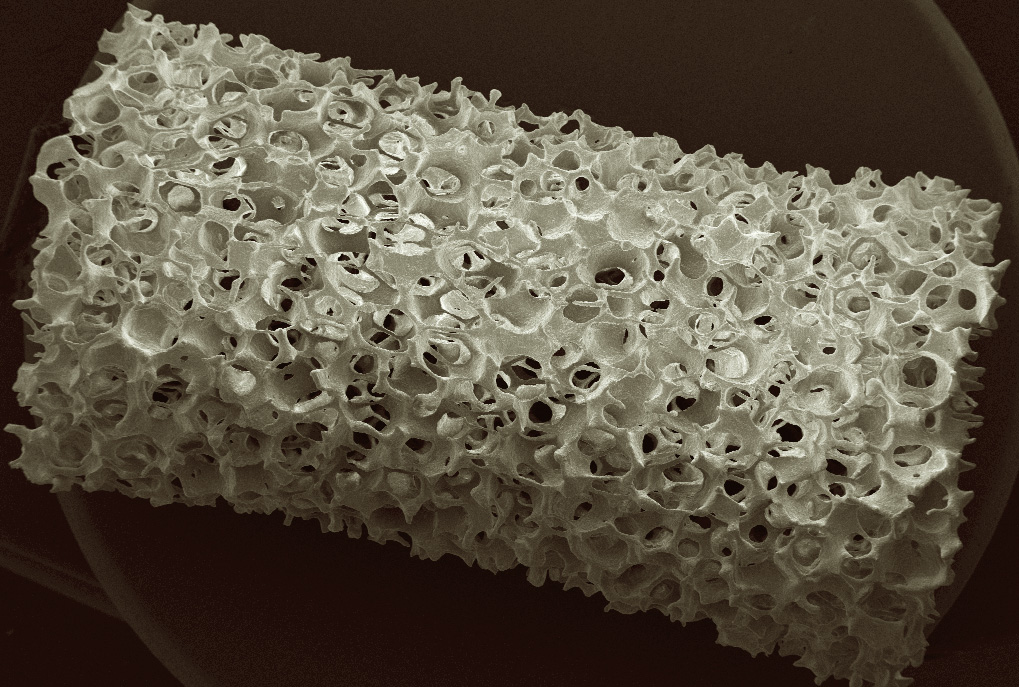
Scanning electron micrograph of bone scaffold imaged at the University of Sydney
Scanning electron microscopy at Microscopy Australia’s University of Sydney facility provided essential information by revealing the structure and biocompatibility of the materials. In-vivo X-ray microtomography at Microscopy Australia at the University of Adelaide enabled the evaluation of the scaffold’s bone-healing potential in animal models.
New stronger bone scaffolds will meet the need for load-bearing bone substitutes.
The University of Sydney has granted an exclusive licence to Australia’s largest manufacturer of orthopaedic devices, Allegra (previously ASDM), to bring the
bone scaffold to market.
In 2021 Allegra Orthopaedics completed a preclinical large animal study to test the performance of the bones scaffold as a spinal fusion device in sheep, whose spines are very similar to human spines. This has been followed up by a paper in Acta Biomaterialia demonstrating that the device has no adverse effects on the patients distant tissues or bodily systems, one of the three major aspects needed for FDA approval.
“This is an exciting project for ASDM and fits our strategy of developing a portfolio of products in orthopaedics. This material is a novel breakthrough technology with huge global potential.”
– Allegra CEO Tom Milicevic
Array
September 22, 2020