A team led by Prof. Shizhang Qiao at the University of Adelaide has overcome this limitation. “We have designed a highly efficient electrode material to catalyse the battery reaction and improve the durability of metal–sulfur batteries,” said Prof. Qiao. “Our new sulfur–metal battery can be charged and discharged at least 10,000 times,” he added.
The researchers have optimised the atomic structure for molybdenum nitride anodes to significantly increase the cycling durability of metal–sulfur batteries.
In their demonstration, the research team used atomic-resolution scanning transmission electron microscopy (STEM) at our University of Adelaide facility to observe how changes in the atomic structure of different molybdenum nitride anode materials (Mo5N6, MoN, and Mo2N) impacted the performance of room temperature sodium–sulfur batteries.
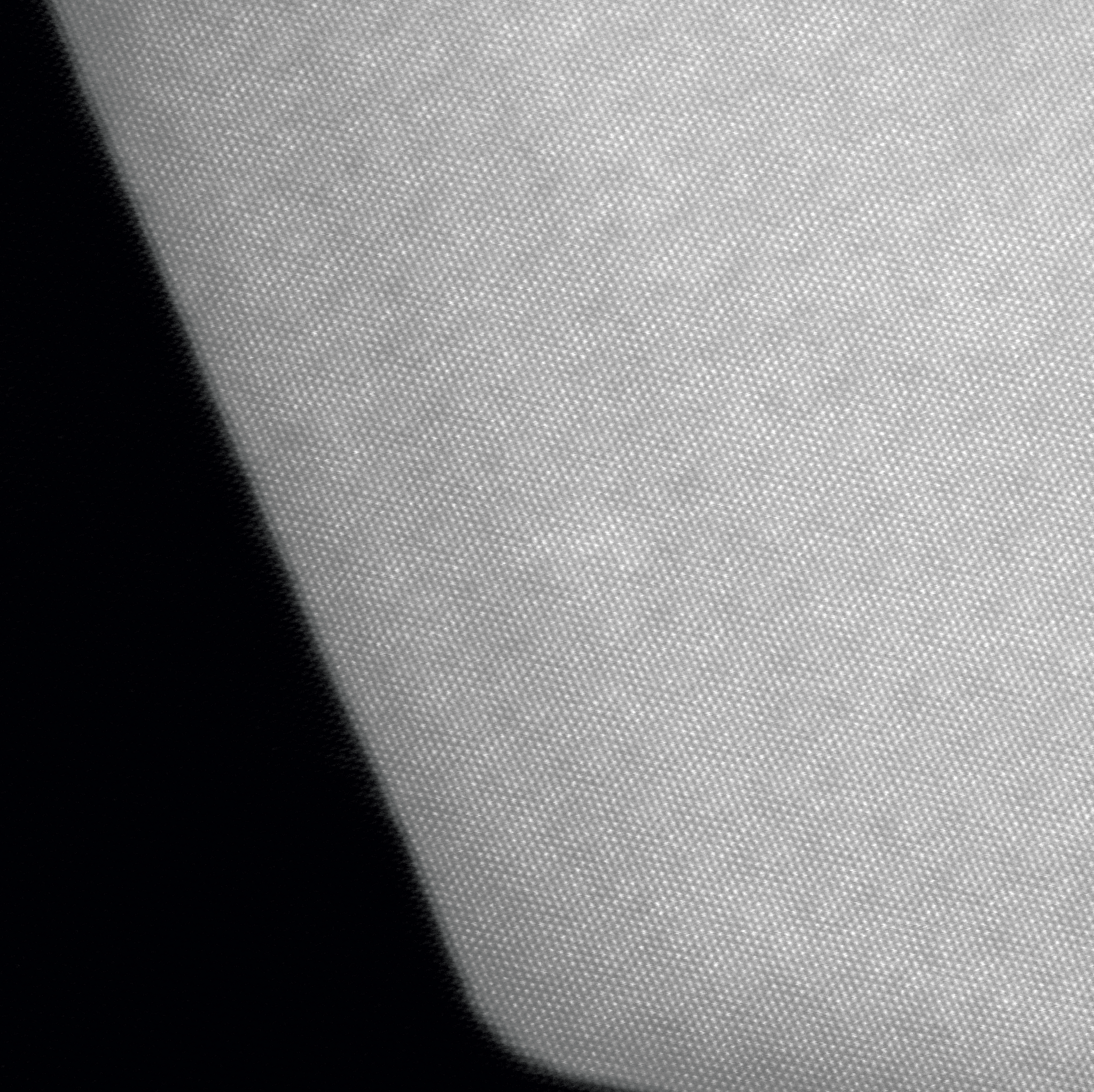
Atomic resolution STEM image of the new electrode material.
They found that despite containing the same elements, the changes in atomic structure between the different molybdenum nitrides had a significant impact on battery efficiency. By choosing the most compatible molybdenum nitride structure, the team created a sodium–sulfur battery that could be cycled over 10,000 times.
This new methodology can now be applied to other metal–sulfur battery types, opening the door to the next-generation of lighter and cheaper batteries, providing long-term storage to help mitigate climate change.
C. Ye et al., Nature Communications 2022
DOI: 10.1038/s41467-021-27551-7
Image: Atomic resolution STEM image of the new electrode material.
Array
May 3, 2023